Today, Karl Giberson continues his six-part series of excerpts from his new book The Wonder of the Universe: Hints of God in a Fine-Tuned World . Karl Giberson, noted speaker and writer about the intersection of Christian faith and science (see first and second posts). (See his complete bio here.)
Today, Karl Giberson continues his six-part series of excerpts from his new book The Wonder of the Universe: Hints of God in a Fine-Tuned World . Karl Giberson, noted speaker and writer about the intersection of Christian faith and science (see first and second posts). (See his complete bio here.)
In this third excerpt, Giberson tells us about how water comes from exploding stars and, if the conditions are just right, exists in liquid form, which–if I remember my grade school science classes–is necessary for life.
Excerpt #3: The Wonder of Water
Large stars near the end of their lives regularly explode as a matter of course. With the force of a billion atomic bombs they strew their contents over unimaginably vast regions of space. It is, of course, a once in a lifetime event for the star—a literal going out with a bang. And even though recorded history is just a few thousand years long—and stars live for billions of years—we have some examples of such explosions that were noted by careful observers.
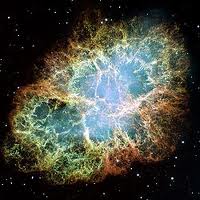
In A.D. 1054 what is now the Crab Nebula exploded in a flash of light bright enough to be seen in daylight for weeks. Astronomers in Korea, China, Japan, North America and the Middle East all recorded the supernova, as it is now called, although Europeans did not. It seems that Europeans, convinced that the heavens were perfect and unchanging, managed to delude themselves into not seeing this new star, which must surely have been quite visible.
The great Danish astronomer Tycho Brahe witnessed another supernova in 1572. Like his predecessors, he could not believe that such a dramatic change in the heavens was possible, but, apparently unlike his predecessors, he had enough confidence in his observations to know that he was seeing something remarkable. Brahe’s protégé, Johannes Kepler, witnessed another supernova in 1604, and then there were no more visible from earth until 1987, when a star exploded in a nearby galaxy known as the Large Magellanic Cloud.
A supernova explosion fills a massive region of space with the elements created inside the star; the powerful explosion, though, follows known laws of physics as it distributes its contents about the universe. A vast cloud of chemically enriched material, trillions of miles in diameter, results from the event—an event absolutely critical for enabling life.
The grand cloud that results from the supernova resembles the original cloud out of which the star formed in the first place, with one important difference—it contains a substantial roster of different materials, and not just hydrogen and helium. This time around gravity has more to work with, beginning again to gather the material in the huge cloud back into balls. The largest chunk at the center becomes another star—one that starts out with heavier elements, in addition to hydrogen. It is the ultimate recycling project, but, unlike recycling on earth, the atoms getting recycled remain in mint condition, no matter how many times they are used.
Some of the smaller balls end up orbiting about the second-generation star. These smaller balls contain many different atoms, and some of them have a curious molecular combination of hydrogen and oxygen. In most parts of the universe these molecules are in the form of a solid. In the others they are a gas. But on balls that are exactly the right distance from the central star, the molecules are liquid, an all-purpose, seemingly magical liquid called water.
Water is found in several places in our solar system. Hydrogen is the most common element in the universe, and while oxygen is far less common it is readily available to combine with hydrogen and form water. Water in the form of ice is a major component in comets and can be found in trace quantities in the atmosphere of Venus, under the surface of Mars and possibly even on some of Jupiter’s moons.
(We have to keep in mind, however, that more than 99 percent of the mass of the solar system is in the sun, so the distribution of elements elsewhere is almost irrelevant from the perspective of the solar system as a whole. The earth has a lot of water, but the earth is a tiny, insignificant speck compared to the sun. And because the water tends to cover so much of the surface, it is easy to overestimate the total amount. Astronomers are not sure exactly where the water on the earth came from. Constructing the early history of our solar system is an enormous challenge.)
From a purely scientific point of view, water is a molecule like any other—and there are lots of molecules. The laws of physics and chemistry describe its behavior, and there are no deep mysteries embedded in its familiar structure. But the laws of physics and chemistry conspire to make water unusual in ways that are critically important for life.
Most peculiarly, water expands rather than contracts when it freezes. This makes ice lighter than water, so it floats. Floating ice insulates the water beneath it from the cold temperatures of winter. Absent this layer of insulation bodies of water all over the earth would freeze solid. If ice were heavier than water, the layer of ice that formed on the top would sink to the bottom and another layer would freeze on top and sink until the entire body of water was a solid piece of ice. This would kill almost every life form in the water.
Water is also an effective solvent. Waste products from our bodies dissolve readily in water and can then easily be expelled. But wait—as they say on television—there is more. Water is also a remarkable coolant capable of absorbing heat and carrying it away from our bodies in the form of sweat. And water stores heat in our bodies, helping keep us warm in cold weather. Magical.
 The creation story in Genesis records that God gathered the waters. In the King James Version that I read as a child it says, “God said, Let the waters under the heaven be gathered together unto one place, and let the dry land appear: and it was so.” In ways that the original readers of Genesis could never have imagined, the gathering of the waters was a cosmic process that took billions of years and involved all the laws of physics and chemistry. The water that we take for granted that covers so much of our planet and makes up so much of our bodies was forged in the nuclear furnace of a star that exploded in the suburbs of the Milky Way galaxy billions of years ago.
The creation story in Genesis records that God gathered the waters. In the King James Version that I read as a child it says, “God said, Let the waters under the heaven be gathered together unto one place, and let the dry land appear: and it was so.” In ways that the original readers of Genesis could never have imagined, the gathering of the waters was a cosmic process that took billions of years and involved all the laws of physics and chemistry. The water that we take for granted that covers so much of our planet and makes up so much of our bodies was forged in the nuclear furnace of a star that exploded in the suburbs of the Milky Way galaxy billions of years ago.
That water now cycles endlessly through the life process here on earth—cooling, cleansing and nurturing us. It irrigates our crops, nourishes our livestock, cleans our clothes and gets turned into snow at ski resorts. In those parts of the world where it is plentiful, clean and fresh, we take it for granted and play with it. In Quebec City they construct a hotel out of ice every winter to attract tourists and invite hardy souls to hold their weddings there, wearing parkas and snow boots. We think nothing of using thousand of gallons so our lawns will be green rather than brown in the heat of summer. Water is like air—plentiful and useful.
In parts of the world where fresh water is rare, its value is more apparent. There is a school in Bulawayo, Zimbabwe where children used to walk a quarter mile during their breaks to get a drink of water. I used to walk to the hallway to get a drink when I was in school. World Vision, one of many organizations helping with water problems around the world, installed a well near the school that the children now use to get water. On school days a group of laughing, happy children can be seen working the oversized pump that takes several of them to manage. The water that emerges from its modest faucet is welcomed in ways that few North Americans can appreciate.
For those school children the water is simply a welcome part of their diet and lifestyle now. Some of the children that stay in school and go on to university will eventually discover that the precious fluid summoned from beneath the earth by a few children cranking on a lever was created billions of years ago, deep in the heart of a star, via processes of unimaginable subtlety. Those that have learned to worship God will no doubt marvel and give thanks.
Water exists because the universe has a set of laws that guide its steady development from the big bang into the present. If we suppose that water and the life it enables are of no consequence, then we can dismiss these laws as irrelevant. On the other hand, if we believe that God is the creator of life and that life has a purpose, then these laws take on a new character. If God is the Creator, then these laws exist because God created them. And these laws work because God upholds them from moment to moment. Viewed by these lights, the origin of water and life are creation events, intentionally enabled by the Creator of the universe.
Next post: Excerpt #4: The Reliability of Science